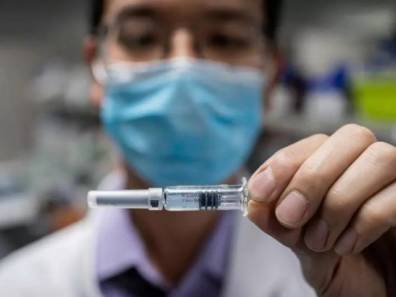
Pakistan has started phase three trials of a vaccine against Covid-19.
In a tweet on Tuesday, Minister for Planning Asad Umar said the vaccine has been developed by a Chinese company.
[adinserter block= “3”]
He said forty thousand people would participate in this trial in seven countries, of which eight to ten thousand will be Pakistanis. The initial results were expected in four to six months.
Falling COVID-19 infection numbers in Pakistan will not affect a Phase 3 clinical trial for a potential vaccine. The trial of China’s CanSino Biologics was expected this month.
Pakistan’s drug regulator last month gave the go-ahead for the country’s first Phase 3 clinical trial for CanSino’s candidate, Ad5-nCoV. It will be led by the National Institute of Health (NIH) and pharmaceutical company AJM – the local representative of CanSino.
Pakistan begins trials of a vaccine against COVID-19
Special Assistant on National Health Services Dr. Faisal Sultan described the trial as a key step to build the country’s capacity in preparing other vaccines against different diseases.
He briefing the media persons in Islamabad on Tuesday.
Meanwhile, Pakistan is now in the list of countries that are conducting clinical trials of the COVID-19 vaccine.
The special assistant expressed the confidence that this vaccine would be safe and effective. Moreover, he said it would benefit not only Pakistan but the peoples around the world.
Officials are positive that the vaccines safe. Meanwhile, it was now being administered to the security personnel in China.
Daily positive cases in Pakistan peaked at more than 6,000 in June. However, the cases have since fallen sharply, with only 426 confirmed new cases detected on September 8; taking its total to 299,659 and 6,359 deaths.
[adinserter block= “4”]
Pakistan, a country of more than 220 million people, has been testing between 20,000 to 30,000 daily.
What do you think of this story? Let us know in the comments section below!







